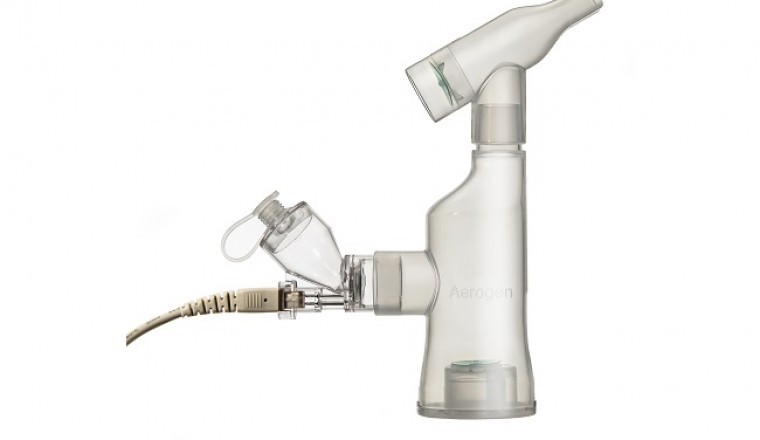
- Synairgen’s SNG001 among the many potential treatments and vaccines to be delivered via Aerogen’s aerosol drug delivery system
- Adds to the thousands of COVID-19 patients already safely and effectively treated with Aerogen’s Solo aerosol drug delivery system in the intensive care unit 1
- Aerogen technology is featured in guidelines and papers on the treatment of COVID-19 patients across the globe 2,3,4
GALWAY, Ireland--(BUSINESS WIRE/AETOSWire)-- Aerogen, the global leader in aerosol drug delivery, today announced details of a broad collaborative effort to support pharmaceutical companies from around the world as they work to develop COVID-19 vaccines and treatments. To date, more than ten million patients globally have been treated safely and effectively with Aerogen’s drug delivery technology 1,5–7. As the global scientific community rallies against the pandemic, there has been unprecedented interest in inhaled delivery of antiviral drugs, leading to Aerogen’s involvement in multiple COVID-19 drug development initiatives with leading pharmaceutical companies and prominent academic groups.
Aerogen’s closed-circuit nebulizer technology, which addresses key concerns around safety and improves patient outcomes5–8, is being used in hospitals across the globe to deliver aerosolized medication to critically-ill ventilated COVID-19 patients1. Aerogen was quick to anticipate the critical role that development of new inhaled drugs will ultimately play in the COVID-19 response, and in March of this year formed a COVID response unit to support projects researching potential treatments and vaccines. This response unit is now working with pharmaceutical companies worldwide to ensure safe delivery of inhaled therapies. Several of these collaborations are already in clinical trials, with others on track to enter studies on moderately and severely ill COVID-19 patients over the weeks and months ahead.
In one such collaboration, Aerogen has signed an agreement with Synairgen plc, a Southampton, UK-based biotechnology company, to provide the market-leading Aerogen® Solo/Ultra nebulizer system for delivery of SNG001 directly into the lungs of COVID-19 patients. SNG001 is an inhaled interferon beta that stimulates the innate immune system. Initial investigation of SNG001 as a potential COVID-19 treatment has been promising – hospitalized patients receiving SNG001 were at reduced risk of developing severe disease and more than twice as likely to recover to the ‘no limitation of activities’ level on the ordinal scale over the course of treatment9,10.
“Aerogen is a highly regarded global company known for providing safe and effective aerosol drug delivery,” said Richard Marsden, CEO of Synairgen. “Ensuring that SGN001 is paired with optimal delivery technology is a vital component of our work to bring this potential treatment to market at scale. Aerogen is our choice because of its proven reputation for drug delivery efficiency and reliability, suitability for use with a wide range of ventilatory support modalities, established high-volume manufacturing and prior regulatory approvals across the globe.”
“In the early days of the pandemic, hospitals were discouraged from using any type of aerosol for COVID-19 treatment – which is understandable given the nature of the virus,” said John Power, CEO and Founder of Aerogen. “Now, it’s clear to health systems worldwide that aerosol drug delivery can be done with improved safety but is an absolute necessity for managing this global crisis. COVID-19 has only reinforced the important role Aerogen plays in safely and effectively delivering treatments to patients across the world, and we’re proud to work with innovators like Synairgen as part of the research and development process for potential COVID-19 vaccines and treatments.”
The Aerogen Solo is a closed-system, single patient use (vibrating mesh) aerosol drug delivery technology offering superior performance across all hospital ventilation modalities5–7,11. Designed for the safety of both the patient and the caregiver, Aerogen’s closed-circuit design enables the only global aerosol drug delivery system which mitigates the transmission of patient-generated infectious aerosol during ventilation12,13. Aerogen is committed to working with pharmaceutical companies worldwide to meet the current global demand for inhaled delivery of antiviral drugs and offers industry-leading expertise and technology in combination with a proven track record in the development of inhalation therapeutics. For more information on Aerogen’s products, please visit www.aerogen.com/products.
For more information on Aerogen’s role in the fight against COVID-19, www.aerogen.com/covid-19.



















Facebook Conversations
Disqus Conversations